Crech Center needs an energetic Executive Director
Please visit our home page for an overview of what we have done … and are doing.
The Center for Research on Environmental Chemicals on Humans is an IRS-approved 501(c)(3) non-profit corporation and needs an executive director who wants to make a difference.
So far, all of our energies have gone into the scientific research and planning to finish the Stealth Syndromes Human Study ??? the first controlled, reproducible human dietary intervention study… ever!
If successful, we will offer consumers and their healthcare providers new, reliable ways to help reduce cancer, Alzheimer’s, obesity, Type 2 Diabetes and other “modern syndromes” that are at epidemic levels.
Management experience (non-profit or otherwise) would be good, but so would be an intelligent, resourceful, energetic organizer looking for an opportunity to make a real, permanent difference in the lives of everyone.
To see what we have already done … and what you can take into orbit and beyond, head over to?? the Crech Center home page.
If you are interested, email Crech Chairman and study co-investigator Lewis Perdue with your LinkedIn profile URL and/or a resume.
IGG
CRECH Transparency: IRS Filings, Guidestar & More
CRECH Projects
Stealth Syndromes Human Study — Approved by the University of California, San Francisco Medical School’s Committee on Human Research in 2015, this is the controlled first human study of environmental chemicals. This effort is the #1 priority for CRECH.
The Stealth Syndromes Project — This multi-year endeavor began as an effort to clarify environmental risks for a non-scientific audience.
After writing scores of articles based upon the study of hundreds of published, peer-reviewed studies, project founders Perdue and Yeamans-Irwin realized that no adequate science existed that could justify valid decisions based on causation.
They realized that to move beyond non-causal links and associations based on animal studies and epidemiology, they needed to create the missing science. With the the collaboration of UCSF Professor and Victor Reus, that study was developed and approved by the UCSF/CHR.
PharmBlockers — Using existing pharmaceutical evaluation results as a more accurate paradigm for assessing environmental chemical risks — This is a fledgling effort that has resulted from Stealth Syndrome Project research which discovered that some environmental chemicals work in ways that counteract approved pharmaceuticals.
More specifically, those environmental chemicals work in direct opposition to the exact same cellular mechanisms targeted by drugs. As one example, published studies indicate that Bisphenol A promotes resistance to cancer chemotherapy, thus making treatment less effective.?? This is especially significant given that most plastic intravenous tubing leaches BPA and associated environmental chemicals.
This example of a “PharmBlocker” suggests that those environmental chemicals need to be evaluated with the same degree of scrutiny as the pharmaceutical whose effects they harm.
Stealth Syndrome Study, Part 4: How Do Science & Society Benefit?
Part 4 of 4
The following is based on “The Study & Protocol As Approved by The UCSF Medical School CHR.” That’s where you will find the citations, footnotes and scientific details. Also, please note that “Chemicals of Emerging Concern” is synonymous with a subset of “Environmental Chemicals.”
Stealth Syndrome Study, Part 1: Where Did The Idea Come From?
Stealth Syndrome Study, Part 2: How Will The Process Work?
Stealth Syndrome Study, Part 3: Controlling Micronutrients
- First connection established between dietary intervention and health indicators.
- Establishment of a framework to move risk assessment of low-level Chemicals of Emerging Concern beyond traditional toxicological evaluations and toward molecular and epigenetic evaluations.
- Development of techniques to reduce exposure to CECs.
- Emphasis on techniques (#2, above) that can easily and economically be implemented by the average person without significant disruption to daily lives.
- Overall improvement in public health and a potential path to reducing the rising incidence of obesity, Type 2 Diabetes, Alzheimer’s disease and other behavioral disorders, fertility and developmental disorders.
Stealth Syndrome Study, Part 1: Where Did The Idea Come From?
Part 1 of 4
The following is based on “The Study & Protocol As Approved by The UCSF Medical School CHR.” That’s where you will find the citations, footnotes and scientific details. Also, please note that “Chemicals of Emerging Concern” is synonymous with a subset of “Environmental Chemicals.”
Stealth Syndrome Study, Part 2: How Will The Process Work?
Stealth Syndrome Study, Part 3: Controlling Micronutrients
Stealth Syndrome Study, Part 4: How Do Science & Society Benefit?
Every study starts with unanswered questions. The main question underpinning the Stealth Syndrome Study was:
“Could the act of removing environmental chemicals (ECs) from known everyday food, beverage and other exposures, affect the outcomes of standard health markers, such as the blood tests hospitals run every day?”
But getting to that main question came only after other questions popped up.
Why? Part 1
Why had no causal connection had ever been established between low levels of environmental chemicals and human harm?
That question haunted us after three years of analyzing the scientific literature for the Stealth Syndromes Project.
Why? Part 2
Why no causal connection? Because there had never been a human study.
Why? Part 3
Why had there never been a human study? Because of ethical concerns over exposing people to potentially harmful chemicals. (See Of Mice, Humans & Ethics.)
Why? Part 4
What’s the big deal over having a human test with exposing people to the same chemicals that they consume every day? And which the FDA says is safe? We still have no answer to that, and it’s not a testable hypothesis for our study. For more: Why Have Regulators Failed To Regulate?
Why? Part 5
How could we conduct a test on humans that was ethical?
Hypothesis
Those questions led to our original question (above). But to conduct a scientific trial, that question needed to be phrased as a testable hypothesis. The hypothesis of the Stealth Syndrome Study was:
The controlled and stepwise elimination of environmental chemicals known as Chemicals of Emerging Concern (CECs) from the test subject environment will result in measurable changes in serum and urine concentrations of specific chemicals and standard clinical health biomarkers attributable to each class of CEC-containing product.
The Correlation/Causation Conflict Conundrum
Remember that science is rarely as precise as non-scientists have come to expect and frequently falls short of what scientists would like.
Our testable hypothesis is that we can establish a causal relationship between removing a CEC/EC from a human’s environment and a change in a blood profile the medical and scientific community trust as an indication of health.
So, the hypothesized causal connection is: “lower CEC/EC exposure causes changes in urine and blood samples in ways that are established indicators of health.”
Implicit in the hypothesis is that the health indicators will change in ways that indicate an improvement in health. That is not a certainty. This is because the health indicators chosen for the study may not be sensitive enough to produce statistically significant results. That’s an inherent risk in a first-of-a-kind investigation like this one.
Even if the study provides support for the hypothesis — this study will not be able to scientifically state that, “CEC/EC exposure causes cancer, obesity, Type II Diabetes, etc.
However, if the hypothesis is supported, the study can move causation ahead by a great leap — to the point that it may be accurate to state that “CEC/EC exposure causes established changes in trusted medical health markers which are indicators or associates of cancer, obesity, Type II Diabetes, etc.”
Stealth Syndrome Study, Part 2: How Will The Process Work?
Part 2 of 4
The following is based on “The Study & Protocol As Approved by The UCSF Medical School CHR.” That’s where you will find the citations, footnotes and scientific details. Also, please note that “Chemicals of Emerging Concern”?? (CEC) is synonymous with a subset of “Environmental Chemicals.” (EC)
Stealth Syndrome Study, Part 1: Where Did The Idea Come From?
Stealth Syndrome Study, Part 3: Controlling Micronutrients
Stealth Syndrome Study, Part 4: How Do Science & Society Benefit?
Why Focus on Bisphenol A (BPA) & Phthlates As Markers?
This study will focus primarily on BPA as a marker for the presence of CEC/ECs. This is because foods, beverages and other exposure sources contain multiple compounds. That makes the task of identifying which compounds (or synergistic combinations) are responsible for a given health effect impractical for the scope and budget of this study.
Indeed, given the lack of data on the health effects of most chemicals involved, the task would be impossible for the budgets and technical abilities of even the most advanced laboratories. Significantly, even less data is known about combined health effects of the everyday mixtures to which consumers are exposed.
BPA and phthalates have been chosen as markers because the U.S. Centers for Disease Control has found those persistently present in almost all Americans.
Given that the now-well-studied BPA and phthalate compounds are often used together — and always used in combination with other polymers, resins, and product enhancement chemicals — we theorize that they are suitable markers for the presence of other ???bad actors.???
Significantly, any health effects that may be observed from our study will clearly reflect possible synergistic effects from combinations of chemicals since it is impossible for us to know exactly which compounds are in a given product.
Stepwise Reductions
As the footnotes in the study & protocol point out, a number of published papers have measured levels of CEC/ECs in foods, beverages and other sources.
We have analyzed those studies and placed them in a manageable set of categories:
Product Category 1: Food (migration and leaching from packaging)
- Eliminating all products packaged in cans and plastic.
- Use of fresh products when possible.
- Products packed in glass may be substituted.
- Plastic-wrapped dry foods (bread, pasta, etc)
- Plastic-wrapped wet fresh foods (veggies, cheese, meat)
- Plastic storage bags
- Milk, Cheese, dairy products
- Cutting boards
Product Category 2: Food (migration and leaching from preparation stressors)
- Foods with metalized plastic ???crisping??? surfaces (Hot Pockets, frozen pizza)
- Paper or plastic plates, glasses and cups
- Take-out and deli plastic containers of all sorts.
- Restaurant and fast food
- Frozen and similar convenience foods
Product Category 3 (Non-alcoholic beverages, migration and leaching from packaging)
- Filtered tap water versus unfiltered.
- Homes/Offices where the water supply comes via PVC or Pex plastics.
- Beverages in pouches, boxes and ???paper bottles???
- Water in hydration bladders like Camelbak
- Drip coffee maker and Keurig (plastic) as well as the Sodastream
Product Category 4 (skin contact/inhalation)
- Laundry detergents (phthalates, fragrances, surfactants)
- Dish and dishwasher soaps (same as laundry)
- Toothpaste (plastic tube) ??? alternative?
- Toothbrush ??? what are the bristles made of?
- Floss?
- Fitbits, plastic watch bands
- Gore-Tex and other waterproof coatings
- Paper currency
- Receipts
Product Category 5 (Alcoholic Beverages – Non-alcoholic beverages, migration and leaching from packaging, Ethanol known solvent for chemicals)
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
- Wine in plastic pouches/bottles/boxes
- Distilled spirits in glass versus plastic bottles.
- Wine and beer ???on tap???
Product Category 6 (Alcoholic Beverages in glass bottles).
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
Product Category 7: (Dairy products: Consumption – inherent content as purchased – resulting from harvest and processing)
The present study will focus on dairy products as a category for its own abstain/intervention. This is because a recent study found an unexpected increase in phthalates especially in children.
That study theorized this increase was due to their greater consumption of milk than adults.
Investigators in that study theorized that the extensive use of plastics in the milk-production process was responsible for the phthalates increase despite the fact that milk was delivered in glass bottles.
In fact, that study calculated that children were exposed to 183 micrograms/kg/day and noted that level was more than 9X higher than, the EPA oral reference dose of 20 micrograms/kg/day.
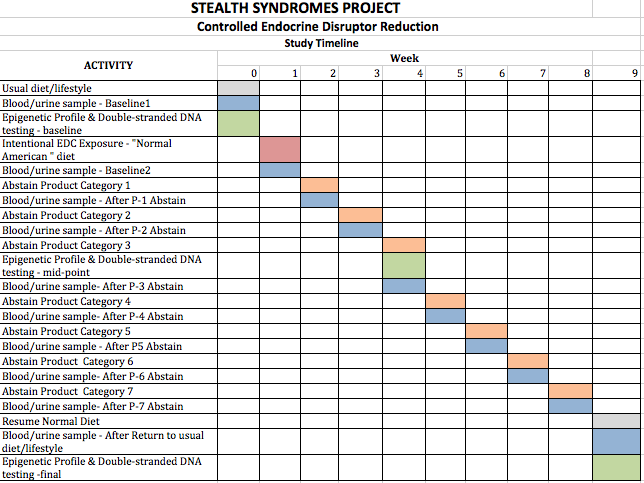
The Schedule
The protocol calls for measurements and stepwise reductions over a nine-week period.
The first week is a baseline period after which health indicators are measured.
The second week, is one in which test participants consume a “typical” diet which will include food and beverages packaged in plastic and heated in the packaging as instructed. The diet will also include foods prepared from cans (tomatoes, vegetables, fruit). In addition they will experience ordinary environmental and contact exposures.
Then, for the next seven weeks, one category after another will be eliminated.
Let???s take one single meal as an example.
Suppose that test subjects eat lasagna every Tuesday evening for dinner for nine weeks.
The first week of the study, the lasagna would be made with commonly used supermarket ingredients such as canned tomatoes and mass-produced ground beef wrapped in plastic, and browned in vegetable oil from plastic containers.
Of concern is the fact established by multiple peer-reviewed studies that??he epoxy linings of more than 70% of food cans leach BPA and other environmental chemicals. The same is true for the plastic used to package ground beef and to bottle vegetable oil.
The second week of the study, the recipe is the same, but instead of cans, tomatoes packed in glass jars would be used in the lasagna. Traces of surfactants used in the washing of the jars may exert some biological action, but that is unknown.
The third week of the study, the recipe remains the same as week two except that lasagna is made with fresh tomatoes.
The fourth week of the study, the recipe remains the same as week three, but the lasagna is made with fresh organic tomatoes.
The fifth week of the study, the recipe remains the same as week four except the lasagna is made with fresh organic tomatoes and freshly ground beef that has never been wrapped in plastic.
The sixth week of the study, the the recipe remains the same as week five except lasagna is made with fresh organic tomatoes,??freshly ground beef that has never been wrapped in plastic and cheeses never exposed to plastic wrapping.
In weeks 7-9 the recipe remains the same as week six. These three final weeks involve the elimination of BPA from non-food and beverage exposures with the result that subjects will consume meals identical to those in week 6.
In order to assure best compliance among test subjects, all meals will be standardized, prepared in advance, packaged in glass, frozen and microwavable.
Snacks and other consumables will be similarly standardized.
Test subjects will engage in real-time, daily logging of everything they eat, drink use, or apply to their bodies.
Health Indicator Measurements
- Weekly blood profile as direct clinical health-linked proxies for CEC body burden.
- Weekly blood profile and urine measurements of Bisphenol A and phthalates for correlation with blood biomarkers.
- Monthly double-stranded DNA break levels.
- Monthly epigenetic profiles of specific methylation locations known to be associated with cancer, obesity, aging, infertility, or Alzheimer???s disease.
We propose using specific elements of standard blood profiles that can provide direct health assessments of inflammation, glucose tolerance, lipid and cholesterol levels and similar well-established indicators.
Rationale For Selection of Specific Clinical Blood Tests
Preliminary universe of tests based on known cellular and biological mechanisms of BPA, phthalates and other CECs. To be narrowed down in consultation with a qualified hematologist.
- Estrogenic activity
- Anti-androgenic activity
- Oxidative stress / inflammation
- Glucose metabolism
- Insulin resistance
- Adipocyte functioning
- Epigenetic alterations
- Interference with Mitosis (centrioles)
- CDK5 effects (thyroid cancer)
- Prostate cancer (PSA levels)
- Accelerated cell proliferation and decreased apoptosis
- Affects on G-Protein Coupled Receptors
- WBC
- Cytokines:Il-1,6,8,10
- TNF alpha
- CRP
- BDNF, VEGF,IGF-BP#3,EGF,FGF,FGF-2?,NGF
- ESR
- F2 isoprostanes
- Cholesterol/HDL/triglycerides
- Hormones:cortisol,prolactin,GH,adiponectin,ghrelin,leptin,insulin, fasting glucose,NPY
- Vit E,C,D
- Fibrinogen
- Cell adhesion molecules:VCAM-1,ICAM
- Oxidative stress markers:glutthione peroxidase,superoxide dismutase,nitric oxide,
- Human methylation 450 bead chip
- telomere length; telomerase
Stealth Syndrome Study, Part 3: Controlling Micronutrients
Part 3 of 4
The following is based on “The Study & Protocol As Approved by The UCSF Medical School CHR.” That’s where you will find the citations, footnotes and scientific details. Also, please note that “Chemicals of Emerging Concern”?? (CEC) is synonymous with a subset of “Environmental Chemicals.” (EC)
Stealth Syndrome Study, Part 1: Where Did The Idea Come From?
Stealth Syndrome Study, Part 2: How Will The Process Work?
Stealth Syndrome Study, Part 4: How Do Science & Society Benefit?
One of the study’s toughest issues is?? the considerable effort needed to the control for potential confounding factors resulting from the epigenetic activity of methyl-contributing micronutrients (such as folate and genistein among others).
Epigenetic means that the activity of a gene is changed without an underlying change (mutation) in a gene. A methyl group is an molecule composed of three hydrogen atoms connected to a carbon atom.
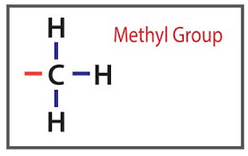
Methyl groups are common in cells and like to form compounds. They can attach themselves to specific locations in the DNA and act in an epigenetic manner to change how the gene expresses itself.
Published work (references at the bottom) indicate that methyl-contributing micronutrients have demonstrated the ability to??significantly alter the known epigenetic effects of BPA. While those studies have been conducted with murine models, the possibility of parallel human effects unknown and could be of substantial concern.
As a result, the possible confounding factors of such micronutrients demand that the study determine reference levels of BPA and that of methyl-contributing micronutrients in the test subject diets on a meal-by-meal basis.
Instead of trying to establish pre-preparation levels of potentially confounding compounds in the food recipes, we propose establishing post-preparation concentrations in an exemplar of each meal which can establish reference points to examine what may be confounding results.
This would require the maceration of one exemplar of each standardized meal and the measurement of levels of BPA and methyl-contributing micronutrients in the supernatant. This would also continue for food and beverages consumed in weeks 7-9 and thus provide a quality control for resulting measurements.
Establishing the concentrations of methyl-contributing micronutrients, and pesticide residues in each meal offers a method to that can be used to control for those confounding factors.
In addition, tracking BPA in each meal may also point to previously unknown sources, especially if unexpected concentrations are found in the meals of weeks five through nine.