Part 2 of 4
The following is based on “The Study & Protocol As Approved by The UCSF Medical School CHR.” That’s where you will find the citations, footnotes and scientific details. Also, please note that “Chemicals of Emerging Concern”?? (CEC) is synonymous with a subset of “Environmental Chemicals.” (EC)
Stealth Syndrome Study, Part 1: Where Did The Idea Come From?
Stealth Syndrome Study, Part 3: Controlling Micronutrients
Stealth Syndrome Study, Part 4: How Do Science & Society Benefit?
Why Focus on Bisphenol A (BPA) & Phthlates As Markers?
This study will focus primarily on BPA as a marker for the presence of CEC/ECs. This is because foods, beverages and other exposure sources contain multiple compounds. That makes the task of identifying which compounds (or synergistic combinations) are responsible for a given health effect impractical for the scope and budget of this study.
Indeed, given the lack of data on the health effects of most chemicals involved, the task would be impossible for the budgets and technical abilities of even the most advanced laboratories. Significantly, even less data is known about combined health effects of the everyday mixtures to which consumers are exposed.
BPA and phthalates have been chosen as markers because the U.S. Centers for Disease Control has found those persistently present in almost all Americans.
Given that the now-well-studied BPA and phthalate compounds are often used together — and always used in combination with other polymers, resins, and product enhancement chemicals — we theorize that they are suitable markers for the presence of other ???bad actors.???
Significantly, any health effects that may be observed from our study will clearly reflect possible synergistic effects from combinations of chemicals since it is impossible for us to know exactly which compounds are in a given product.
Stepwise Reductions
As the footnotes in the study & protocol point out, a number of published papers have measured levels of CEC/ECs in foods, beverages and other sources.
We have analyzed those studies and placed them in a manageable set of categories:
Product Category 1: Food (migration and leaching from packaging)
- Eliminating all products packaged in cans and plastic.
- Use of fresh products when possible.
- Products packed in glass may be substituted.
- Plastic-wrapped dry foods (bread, pasta, etc)
- Plastic-wrapped wet fresh foods (veggies, cheese, meat)
- Plastic storage bags
- Milk, Cheese, dairy products
- Cutting boards
Product Category 2: Food (migration and leaching from preparation stressors)
- Foods with metalized plastic ???crisping??? surfaces (Hot Pockets, frozen pizza)
- Paper or plastic plates, glasses and cups
- Take-out and deli plastic containers of all sorts.
- Restaurant and fast food
- Frozen and similar convenience foods
Product Category 3 (Non-alcoholic beverages, migration and leaching from packaging)
- Filtered tap water versus unfiltered.
- Homes/Offices where the water supply comes via PVC or Pex plastics.
- Beverages in pouches, boxes and ???paper bottles???
- Water in hydration bladders like Camelbak
- Drip coffee maker and Keurig (plastic) as well as the Sodastream
Product Category 4 (skin contact/inhalation)
- Laundry detergents (phthalates, fragrances, surfactants)
- Dish and dishwasher soaps (same as laundry)
- Toothpaste (plastic tube) ??? alternative?
- Toothbrush ??? what are the bristles made of?
- Floss?
- Fitbits, plastic watch bands
- Gore-Tex and other waterproof coatings
- Paper currency
- Receipts
Product Category 5 (Alcoholic Beverages – Non-alcoholic beverages, migration and leaching from packaging, Ethanol known solvent for chemicals)
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
- Wine in plastic pouches/bottles/boxes
- Distilled spirits in glass versus plastic bottles.
- Wine and beer ???on tap???
Product Category 6 (Alcoholic Beverages in glass bottles).
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
Product Category 7: (Dairy products: Consumption – inherent content as purchased – resulting from harvest and processing)
The present study will focus on dairy products as a category for its own abstain/intervention. This is because a recent study found an unexpected increase in phthalates especially in children.
That study theorized this increase was due to their greater consumption of milk than adults.
Investigators in that study theorized that the extensive use of plastics in the milk-production process was responsible for the phthalates increase despite the fact that milk was delivered in glass bottles.
In fact, that study calculated that children were exposed to 183 micrograms/kg/day and noted that level was more than 9X higher than, the EPA oral reference dose of 20 micrograms/kg/day.
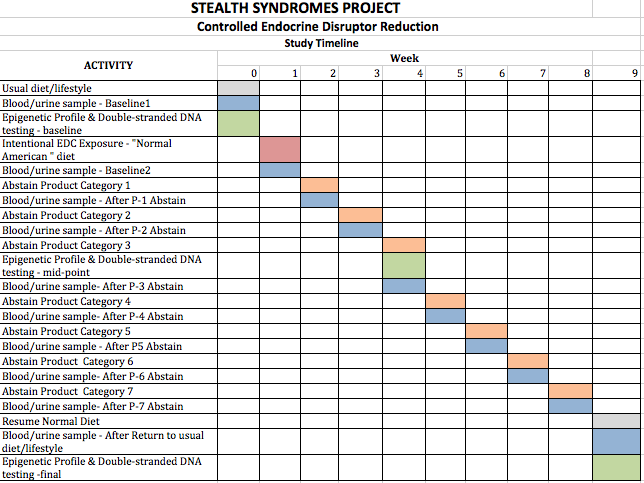
The Schedule
The protocol calls for measurements and stepwise reductions over a nine-week period.
The first week is a baseline period after which health indicators are measured.
The second week, is one in which test participants consume a “typical” diet which will include food and beverages packaged in plastic and heated in the packaging as instructed. The diet will also include foods prepared from cans (tomatoes, vegetables, fruit). In addition they will experience ordinary environmental and contact exposures.
Then, for the next seven weeks, one category after another will be eliminated.
Let???s take one single meal as an example.
Suppose that test subjects eat lasagna every Tuesday evening for dinner for nine weeks.
The first week of the study, the lasagna would be made with commonly used supermarket ingredients such as canned tomatoes and mass-produced ground beef wrapped in plastic, and browned in vegetable oil from plastic containers.
Of concern is the fact established by multiple peer-reviewed studies that??he epoxy linings of more than 70% of food cans leach BPA and other environmental chemicals. The same is true for the plastic used to package ground beef and to bottle vegetable oil.
The second week of the study, the recipe is the same, but instead of cans, tomatoes packed in glass jars would be used in the lasagna. Traces of surfactants used in the washing of the jars may exert some biological action, but that is unknown.
The third week of the study, the recipe remains the same as week two except that lasagna is made with fresh tomatoes.
The fourth week of the study, the recipe remains the same as week three, but the lasagna is made with fresh organic tomatoes.
The fifth week of the study, the recipe remains the same as week four except the lasagna is made with fresh organic tomatoes and freshly ground beef that has never been wrapped in plastic.
The sixth week of the study, the the recipe remains the same as week five except lasagna is made with fresh organic tomatoes,??freshly ground beef that has never been wrapped in plastic and cheeses never exposed to plastic wrapping.
In weeks 7-9 the recipe remains the same as week six. These three final weeks involve the elimination of BPA from non-food and beverage exposures with the result that subjects will consume meals identical to those in week 6.
In order to assure best compliance among test subjects, all meals will be standardized, prepared in advance, packaged in glass, frozen and microwavable.
Snacks and other consumables will be similarly standardized.
Test subjects will engage in real-time, daily logging of everything they eat, drink use, or apply to their bodies.
Health Indicator Measurements
- Weekly blood profile as direct clinical health-linked proxies for CEC body burden.
- Weekly blood profile and urine measurements of Bisphenol A and phthalates for correlation with blood biomarkers.
- Monthly double-stranded DNA break levels.
- Monthly epigenetic profiles of specific methylation locations known to be associated with cancer, obesity, aging, infertility, or Alzheimer???s disease.
We propose using specific elements of standard blood profiles that can provide direct health assessments of inflammation, glucose tolerance, lipid and cholesterol levels and similar well-established indicators.
Rationale For Selection of Specific Clinical Blood Tests
Preliminary universe of tests based on known cellular and biological mechanisms of BPA, phthalates and other CECs. To be narrowed down in consultation with a qualified hematologist.
- Estrogenic activity
- Anti-androgenic activity
- Oxidative stress / inflammation
- Glucose metabolism
- Insulin resistance
- Adipocyte functioning
- Epigenetic alterations
- Interference with Mitosis (centrioles)
- CDK5 effects (thyroid cancer)
- Prostate cancer (PSA levels)
- Accelerated cell proliferation and decreased apoptosis
- Affects on G-Protein Coupled Receptors
- WBC
- Cytokines:Il-1,6,8,10
- TNF alpha
- CRP
- BDNF, VEGF,IGF-BP#3,EGF,FGF,FGF-2?,NGF
- ESR
- F2 isoprostanes
- Cholesterol/HDL/triglycerides
- Hormones:cortisol,prolactin,GH,adiponectin,ghrelin,leptin,insulin, fasting glucose,NPY
- Vit E,C,D
- Fibrinogen
- Cell adhesion molecules:VCAM-1,ICAM
- Oxidative stress markers:glutthione peroxidase,superoxide dismutase,nitric oxide,
- Human methylation 450 bead chip
- telomere length; telomerase