The Committee on Human Research (CHR) at the University of San Francisco Medical School has approved the Stealth Syndromes experimental protocol.
This study approval allows Stealth Syndromes co-founder Lewis Perdue to become the test subject for a “proof of concept” study to determine if standard clinical blood tests can be used to detect physiological changes that may be caused by the absorption of environmental chemicals, including endocrine disrupting compounds.
The CHR gave its go-ahead for the study on on Nov. 11, 2015.
The Stealth Syndromes Project is very fortunate (and grateful) to have had the guidance and institutional mentorship of UCSF Professor Victor Reus, M.D.
Dr. Reus is an internationally renowned and widely published biomedical scientist whose interests include neurobiology and behavior as well as the genetic origins of psychiatric disorders.
In the coming days, I will be writing numerous posts that describe the various facets of the study and examine them in more detail. Those will look at the value of a first “proof of concept study,” and what makes this study different from the hundreds of others dealing with environmental chemicals.
iRIS – Let a Thousand Computer Forms Blossom
Study submissions at UCSF are entirely electronic. Everything is submitted through the secure, web-based iRIS system which provides scores of forms in a relatively intuitive framework.
iRIS centralizes all aspects of study, makes sure that all the relevant parts of a study are included, assures compliance with human research protections, and makes all that available to the university compliance process without needing paper versions.
The complexity of the iRIS system lies somewhere between IRS income tax forms and the paperwork associated with buying and mortgaging a new house.
The following is from the iRIS FAQs:
iRIS is a web-based system that enables online application submission, real-time submission tracking, review, post-approval compliance activities, and data management. The system also functions as a document repository, providing investigators with easy access to submission records and study documents.
The committees that will conduct their reviews using the iRIS system include the:
- Committee on Human Research (CHR),
- the CTSI Clinical Research Services (CRS) Advisory Committees, and
- the Human Gamete, Embryo, and Stem Cell Research Committee (GESCR committee).
Investigators can use the system anywhere they have Internet access, helping to connect faculty, researchers, students and partners around the world. The iRIS system also has expedited the CHR review and approval process. In addition, prior to the implmenetation of iRIS, the CHR received about 5 million pieces of paper each year. The electronic system has helped us eliminate this waste and supports UCSF’s efforts to incorporate environmentally-friendly practices across campus.
Summarizing the study submission
The text below is taken directly from the UCSF iRIS system.
I have included ALL of the sections relevant to the study and omitted only those sections associated with administrative details and the online human research protection training courses I needed to pass in order to qualify as a study co-principal investigator. Correct section numbers from iRIS are used to designate each portion.
10.0 STUDY DESIGN
This is an interventional dietary study to determine the usefulness of carefully selected tests from standard medical blood profiles to indicate health effects resulting from the reduction of specific foods and substances known to contain certain environmental chemicals.
This study is the first to use easily accessed and medically accepted laboratory methods to directly measure health effects of dietary intervention on the reduction of persistent and ubiquitous environmental chemicals. While this is an n=1, proof-of-concept study using co-Principal Investigator Lewis Perdue as the test subject, it expands upon previous studies with a longer observation period, and by designating specific substances (packaged in glass vs plastic or cans, elimination of dermal contact) for intervention instead of general parameters (fresh versus prepared foods).
11.1 HYPOTHESIS
SPECIFIC AIMS:
The primary aim of this study is to determine whether a positive correlation exists between CEC intervention, test subject blood profiles, and CEC levels in serum and urine. Because the significance of CEC urine and serum levels is controversial, the primary aim of this study is to provide a measurement of direct CEC health effects (or lack thereof) using widely available laboratory blood profile indicators.
A secondary aim of this study is to provide a method to correlate potential health effects of CECs with their observed human levels as previously measured by NHANES and other investigations.
12.1 BACKGROUND
INTRODUCTION
The human health effects of low-level concentrations of certain Chemicals of Emerging Concern (CECs) has stirred immense controversy between traditional toxicologists and a more recent, emerging body of scientists grounded in epigenetics and molecular-level effects. Traditional toxicologists insist that current risk evaluations at high concentration levels can be monotonically extrapolated to low concentrations and that a firm No Observed Adverse Effects Level (NOAEL) of safety can be established.
On the other hand, a more recent and growing body of peer-reviewed, published data indicates that many CECs exhibit non-monotonic behavior and present risks to humans at low concentrations. That controversy continues partly because of the lack of controlled human studies and the almost complete absence of investigations into effects of combinations of CECs.
BACKGROUND
Exposure to environmental chemicals in the U.S. is widespread20.
More than 84,000 chemicals are approved for use in the United States today1, and at least 4,000 of those are present in food contact materials2,3,4. The health effects of most of those chemicals is unknown and/or incomplete5..
While controversial, many of these chemicals in low-level concentrations are increasingly classified as endocrine disruptors22,23.
Among chemicals of emerging concern (CEC) are Bisphenol A (BPA) and phthalates, both of which are present in approximately 97% of the U.S. population.6,8 Public concern over the risks from these chemicals have resulted in the reduction of concentrations of some7, but has also led increases in concentrations of substitutes which are also of concern. 39
BPA is used to strengthen and offer heat resistance to common plastics such as polycarbonate. Phthalates are added to plastics for flexibility. Those two compounds are among the most common and widely studied chemicals of emerging concern. For that reason, this study will use them as proxies for overall chemical contamination.
Exposure
BPA and phthalates have become nearly ubiquitous in our environment and can be found in many different products, including the plastic in water bottles and baby bottles, thermal paper for printers, and even in dental sealants and medical devices including intravenous fluid and chemotherapy bags and tubing 8,9,10,1,12,13,14.
In addition, food and beverage packaging are substantial contributors to the CEC burden8,15,16,17,25,26.
Consumers are exposed to many CECs from leaching and migration of chemicals from plastics and other food contact materials.8,14,15,16, 30-37
Other chemicals of concern are deliberately added to consumer and household products such as detergents, cosmetics, lotions, and fragrances38.
Still other contamination may result from the harvest and processing of food products17.
Causes For Concern
Human and animal studies have identified these compounds as contributors to cancer24,40-52,, cardiovascular disorders53-61, obesity62-68, type 2 diabetes69-72, metabolic syndrome73-77, neurological and behavioral disorders including Alzheimer???s Disease78-84, as well as reproductive85-94, and developmental95-102 disorders and allergies103-110.
Specific Exposure Routes
Exposure routes for all products include:
- Migration/leaching of chemicals from packaging materials,
- Deliberate addition of chemicals used as preservatives, flavorings, scents, texture enhancers, coloring agents etc.4,
- Contamination by unknown compounds formed by chemical reactions among multiple intentionally used constituent chemicals18.
Exposure routes for food and beverages specifically include:
- Incidental contamination via migration/leaching of chemicals from harvesting and processing17.
- Home food-handling can also accelerate migration through heating, microwaving, ultraviolet light exposure (including fluorescent lighting) and the contact of oils and alcohols with plastics.
12.2 PRELIMINARY STUDIES
So far, all studies that evaluate potential adverse health effects of CECs by controlled exposure have been done in vitro or in vivo using murine or other non-human models. Despite the fact that all of the CECs in question are nearly ubiquitous in the human environment, ethical concerns have prevented controlled exposure studies. Practical concerns also complicate controlled human exposure studies because ubiquitous exposure to mixtures of CECs make it impossible to create an adequate control population.
Because of that, a small number of interventional dietary studies have been done. These studies have focused on foods and beverages because they constitute major sources of CECs. Dietary interventions are easier to control and offer opportunities to reduce health risks24,27.
Recent dietary interventions16, 17,28 have found significant reductions in the targeted chemicals measured concurrent with study designs to replace pre-prepared meals and other foods with known levels of endocrine disruptors with a fresh, home-prepared diet.
Those interventional studies have been:
- time-limited (3 -16 days),
- involved relatively small numbers of test subjects (20 – 40),,
- imposed very general dietary restrictions (whole diet, fresh foods).
The most significant failing, however, is the failure to connect the reduced levels of CECs to any measurable indication of health benefits.
12.3 REFERENCES
[PARTIAL]
1. EPA. TSCA Chemical Substance Inventory [website]. Washington, DC:U.S. Environmental Protection Agency (updated 13 March 2014). Available http://www.epa.gov/oppt/existingchemicals/pubs/tscainventory/basic.html [accessed 1 July 2015]
2. European Food Safety Authority (EFSA). Report of ESCO WG on non-plastic food contact materials, 2011 [updated 25 July 201116 June 2013]. http://www.efsa.europa.eu/en/supporting/pub/139e.htm
3. Neltner, T.G., Kulkarni, N.R., Alger, H.M., Maffini, M.V., Bongard, E.D., Fortin, N.D., and Olsen, E.D. 2011. Navigating the U.S. Food Additive Regulatory Program. Comprehensive Reviews in Food Science and Food Safety 10:342???68.
4. Muncke, J., Myers, J.P., Scheringer, M., and Porta, M. 2014. Food packaging and migration of food contact materials: will epidemiologists rise to the neotoxic challenge?. Journal of Epidemiology and Community Health 68(7): 592-594.
5. Judson, R., Richard, A., Dix, D. J., Houck, K., Martin, M., Kavlock, R., Dellarco, V., Henry, T., Holderman, T., Sayre, P., Tan, S., Carpenter, T., and Smith, E. 2009. The Toxicity Data Landscape for Environmental Chemicals. Environmental Health Perspectives 117(5), 685???695.
6. National Report on Human Exposure to Environmental Chemicals, U.S. Centers for Disease Control, Fourth report, Updated 2015 Tables, http://www.cdc.gov/exposurereport/index.html, accessed 11 July 2015.
7. Zota, A. R., Calafat, A. M., and Woodruff, T. J. 2014. Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001???2010. Environmental Health Perspectives 122(3), 235???241.
8. Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N., Welshons, W.V. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24: 139-177.
9. Nam, S.H., Seo, Y.M., Kim, M.G. 2010. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 79: 949-952.
10. Biedermann, S., Tschudin, P., and Grob, K. 2010. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and Bioanalytical Chemistry 398: 571-576.
11. Ehrlich, S., Calafat, A.M., Humblet, O., Smith, T., and Hauser, R. 2014. Handling of thermal receipts as a source of exposure of Bisphenol A. JAMA 311(8): 859-860.
12. Kloukos, D., Pandis, N., and Eliades, T. 2013. In vivo bisphenol-A release from dental pit and fissure sealants: A systematic review. Journal of Dentistry 41: 659-667.
13. Duty, S.M., Mendonca, K., Hauser, R., Calafat, A.M., Ye, X., Meeker, J.D., Ackerman, R., Cullinane, J., Faller, J., and Ringer, S. 2013. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics 131(3): 483-489.
14. Yang, C.Z., Yaniger, S.I., Jordan, V.C., Klein, D.J., and Bittner, G.D. 2011. Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environmental Health Perspectives 119(7): 989-996.
15. Bhunia, K., Sablani, S. S., Tang, J. and Rasco, B. 2013. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Comprehensive Reviews in Food Science and Food Safety 12: 523???545.
16. Rudel, R.A., Gray, J.M., Engel, C.L., Rawsthorne, T.W., Dodson, R.E., Ackerman, J.M., Rizzo, J., Nudelman, J.L. and Brody, J.G. 2011. Food packaging and bisphenol A and bis (2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environmental Health Perspectives 119(7): 914-920.
17. Sathyanarayana, S., Alcedo, G., Saelens, B,E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. Journal of Exposure Science and Environmental Epidemiology 23(4): 378-384.
18. Nerin, C., Alfaro, P., Aznar, M., and Dome??o, C. 2013. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Analytica Chimica Acta 775:14???24.
19. Calafat, Antonia M., Ye, X., Wong, L-Y., Reidy, J.A., and Needham, L.L. 2008. Exposure of the US population to Bisphenol A and 4-tertiary-Octylphenol: 2003-2004. Environmental Health Perspectives 116(1): 39-44.
20. Vandenberg, L.N., Chahoud, I., Heindel, J.J., Padmanabhan, V., Paumgartten, F.J., Schoenfelder, G. 2012. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 17:407???434.
21. Thayer, K.A., Doerge, D.R., Hunt, D., Schurman, S.H., Twaddle, N.C., Churchwell, M.I., Garantziotis, S., Kissling, G.E., Easterling, M.R., Bucher, J.R., and Birnbaum, L.S. 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment International 83: 107-115.
22. Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N., and Welshons, W. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24(2): 139-177.
23. Diamanti-Kandarakis, E., Bourguignon, J-P., Giudice, L.C., Hauser, R., Prins, G.S., Soto, A.M., Zoeller, R.T., and Gore, A.C. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews 30(4): 293-342.
24. Ferguson, L.R., Chen, H., Collins, A.R., Connell, M., Damia, G., Dasgupta, S., Malhotra, M., Meeker, A.K., Amedei, A., Amin, A., et al. 2015. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Seminars in Cancer Biology 35: S5-24.
25. Christensen, K.L.Y., Lorber, M., Koslitz, S., Br??ning, T., and Koch, H.M. 2012. The contribution of diet to total bisphenol A body burden in humans: Results of a 48 hour fasting study. Environment International 50: 7-14.
26. Vandenberg, L.N., Hunt, P.A., Myers, J.P., and Vom Saal, F.S. 2013. Human exposures to bisphenol A: mismatches between data and assumptions. Reviews on Environmental Health 28(1): 37-58.
27. Szic, V., Szarc, K., Declerck, K., Vidakovi, M., and Vanden, B.W. 2015. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition?. Clinical Epigenetics 7(1): 33.
28. Ackerman, J.M., Dodson, R.E., Engel, C.L., Gray, J.M., and Rudel, R.A. 2014. Temporal variability of urinary di (2-ethylhexyl) phthalate metabolites during a dietary intervention study. Journal of Exposure Science and Environmental Epidemiology 24(6): 595-601.
29. Calafat, A.M., Longnecker, M.P., Koch, H.M., Swan, S.H., Hauser, R., Goldman, L.R., Lanphear, B.P., Rudel, R.A., Engle, S.M., Teitelbaum, S.L., Whyatt, R.M., and Wolff, M.S. 2015. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environmental Health Perspectives 123(7): A166-8.
30. Bang, D.Y., Kyung, M., Kim, M.J., Jung, B.Y., Cho, M.C., Choi, S.M., Kim, Y.W., Lim, S.K., Lim, D.S., Won, A.J., Kwack, S.J., Lee, Y., Kim, S.K., and Lee, B.M. 2012. Human Risk Assessment of Endocrine Disrupting Chemicals Derived from Plastic Food Containers. Comprehensive Reviews in Food Science and Food Safety 11(5): 453-470.
31. Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 27(1): 132-138.
32. Serrano, S.E., Braun, J., Trasande, L., Dills, R., and Sathyanarayana, S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health 13(1): 43.
33. Rodgers, K.M., Rudel, R.A., and Just, A.C. 2014. Phthalates in Food Packaging, Consumer Products, and Indoor Environments. Toxicants in Food Packaging and Household Plastics: 31-59. **
34. Hayasaka, Y. 2014. Analysis of phthalates in wine using liquid chromatography tandem mass spectrometry combined with a hold-back column: Chromatographic strategy to avoid the influence of pre-existing phthalate contamination in a liquid chromatography system. Journal of Chromatography A 1372: 120-127. **
35. Wagner, M., and Oehlmann, J. 2009. Endocrine disruptors in bottled mineral water: Total estrogenic burden and migration from plastic bottles. Environmental Science and Pollution Research 16(3): 278-286.
36. Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free, hard and clear, thermoplastic resins. Environmental Health 13(1): 103.
37. Vandermeersch, G., Louren??o, H.M., Alvarez-Mu??oz, D., Cunha, S., Diog??ne, J., Cano-Sancho, G., Kwadijk, C., Barcelo, D., Allegaert, W., Bekaert, K., Fernandes, J.O., Marques, A., and Robbens, J. 2015. Environmental contaminants of emerging concern in seafood???European database on contaminant levels. Environmental Research 143(B): 29-45.**
38. Myers, SlL., Yang, C.Z., Bittner, G.D., Witt, K.L., Tice, R.R., and Baird, D.D. 2014. Estrogenic and anti-estrogenic activity of off-the-shelf hair and skin care products. Journal of Exposure Science and Environmental Epidemiology 25(3): 271-277.
39. Rochester, J.R., and Bolden, A.L. 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environmental Health Perspectives 123(7): 643-650.
CANCER
40. Keri, R.A., Ho, S.M., Hunt, P.A., Knudsen, K.E., Soto, A.M., and Prins, G.S. 2007. An evaluation of the evidence for the carcinogenic activity of bisphenol A. Reproductive Toxicology 24: 240-252.
41. Fang, L., Wuptra, K., Chen, D., Li, H., Huang, S.-K., Jin, C., and Yokoyama, K. K. 2014. Environmental-stress-induced Chromatin Regulation and its Heritability. Journal of Carcinogenesis & Mutagenesis 5(1), 22058.
42. Fang, L., Wuptra, K., Chen, D., Li, H., Huang, S.-K., Jin, C., and Yokoyama, K. K. 2014. Environmental-stress-induced Chromatin Regulation and its Heritability. Journal of Carcinogenesis & Mutagenesis 5(1), 22058.
43. Vega, A., Baptissart, M., Caira, F., Brugnon, F., Lobaccaro, J.-M. A., and Volle, D. H. 2012. Epigenetic: a molecular link between testicular cancer and environmental exposures. Frontiers in Endocrinology 3: 150.
44. Tarapore, P., Ying, J., Ouyang, B., Burke, B., Bracken, B., and Ho, S-M. 2014. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PloS ONE 9(3): e90332.
45. Wong, R.L., Wang, Q., Trevi??o, L.S., Bosland, M.C., Chen, J., Medvedovic, M., Prins, G.S., Kurunthachalan, K., Ho, S-M., and Walker, C.L. 2015. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics 10(2): 127-134.
46. Ferguson, L.R., Chen, H., Collins, A.R., Connell, M., Damia, G., Dasgupta, S., Malhotra, M., Meeker, A.K., Amedei, A., Amin, A. et al. 2015. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Seminars in Cancer Biology 35: S5-24.
47. Zhang, Z., Chen, S., Feng, Z., and Su, L.J. 2015. Pregnancy Exposures Determine Risk of Breast Cancer in Multiple Generations of Offspring. In: Environmental Epigenetics. Springer London. pp. 75-103.
48. Gassman, N.R., Coskun, E., Stefanick, D.F., Horton, J.K., Jaruga, P., Dizdaroglu, M., and Wilson, S.H. 2015. Bisphenol A promotes cell survival following oxidative DNA damage in mouse fibroblasts. PloS ONE 10(2): e0118819. **
49. Bishop, K.S., and Ferguson, L.R. 2015. The Interaction between Epigenetics, Nutrition and the Development of Cancer. Nutrients 7(2): 922-947.
50. Kim, Y-S., Hwang, K-A., Hyun, S-H., Nam, K-H., Lee, C-K., and Choi, K-C. 2015. Bisphenol A and Nonylphenol Have the Potential to Stimulate the Migration of Ovarian Cancer Cells by Inducing Epithelial???Mesenchymal Transition via an Estrogen Receptor Dependent Pathway. Chemical Research In Toxicology 28(4): 662-671.
51. Nahta, R., Al-Mulla, F., Al-Temaimi, R., Amedei, A., Andrade-Vieira, R., Bay, S., Brown, D.G., Calaf, G.M., Castellino, R.C., Cohen-Solal, K.A. et al. 2015. Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression. Carcinogenesis 36(S1): S2-S18.
52. Hajjari, M., and Salavaty, A. 2015. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biology & Medicine 12(1): 1.
CARDIOVASCULAR
53. Fang, L., Wuptra, K., Chen, D., Li, H., Huang, S.-K., Jin, C., and Yokoyama, K. K. 2014. Environmental-stress-induced Chromatin Regulation and its Heritability. Journal of Carcinogenesis & Mutagenesis 5(1), 22058.
54. Gao, X., and Wang, H-S.. 2014. Impact of bisphenol A on the cardiovascular system???epidemiological and experimental evidence and molecular mechanisms. International Journal of Environmental Research and Public Health 11(8): 8399-8413.
CARDIAC
55. Ranci??re, F., Lyons, J.G., Loh, V.H, Botton, J., Galloway, T., Wang, T., Shaw, J.E., and Magliano, D.J. 2015. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environmental Health 14(1): 46.
56. Bae, S., and Hong, Y-C. 2015. Exposure to Bisphenol A From Drinking Canned Beverages Increases Blood Pressure Randomized Crossover Trial. Hypertension 65(2): 313-319.
57. Belcher S.M., Chen Y., Yan S., and Wang H.S. 2012. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17??-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology 153: 712???720.
58. Gao X., Liang Q., Chen Y., and Wang H.S. 2013. Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology 154: 4607???4617.
59. Liang Q., Gao X., Chen Y., Hong K., and Wang H.S. 2014. Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environmental Health Perspectives 122 :601???608.
60. Melzer D., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack, P., Luben, R., Khaw, K.T., Wareham, N.J., and Galloway, T.S. 2012. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125: 1482???1490.
61. Yan S., Song W., Chen Y., Hong K., Rubinstein J., and Wang H.S. 2013. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia???reperfusion in female rat hearts. Food and Chemical Toxicology 56: 75???80.
OBESITY
62. Regnier, S.M. and Sargis, R.M. 2014. Adipocytes under assault: Environmental disruption of adipose physiology. Biochimica et Biophysica Acta 1842(3): 520-533.
63. Ellero-Simatos, S., Claus, S.P., Benelli, C., Forest, C., Letourneur, F., Cagnard, N., Beaune, P.H. and de Waziers, I. 2011. Combined Transcriptomic???1H NMR Metabonomic Study Reveals That Monoethylhexyl Phthalate Stimulates Adipogenesis and Glyceroneogenesis in Human Adipocytes. Journal of Proteome Research 10(12): 5493-5502.
64. Marmugi, A., Ducheix, S., Lasserre, F., Polizzi, A., Paris, A., Priymenko, N., Bertrand-Michel, J., Pineau, T., Guillou, H., Martin, P.G., and Mselli-Lakhal, L. 2012. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 55(2): 395-407.
65. Hugo, E.R., Brandebourg, T.D., Woo, J.G., Loftus, J., Alexander, J.W., Ben- Jonathan, N. 2008. Bisphenol A at environmentally relevant doses inhibits adi- ponectin release from human adipose tissue explants and adipocytes. Environmental Health Perspectives 116(12): 1642-1647.
66. Menale, C., Piccolo, M.T., Cirillo, G., Calogero, R.A., Papparella, A., Mita, L., Del Giuduce, E.M., Diano, N., Crispi, S., and Mita, D.G. 2015. Bisphenol A effects on gene expression in children adipocytes: association to metabolic disorders. Journal of Molecular Endocrinology 54(3): 289-303.
67. Savastano, S., Tarantino, G., D???Esposito, V., Passaretti, F., Cabaro, S., Liotti, A., Liguoro, D., Perruolo, G., Ariemma, F., Finelli, C., Bequinot, F., Formisano, P., and Valentino, R. 2015. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. Journal of Translational Medicine 13(1): 1-7.
68. Seidlov??-Wuttke, D., Jarry, H., Christoffel, J., Rimoldi, G., and Wuttke, W. 2005. Effect of bisphenol-A (BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: A 3 month comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology 213: 13-24.
DIABETES
69. Alonso-Magdalena, P., Morimoto, S., Ripoll, C., Fuentes, E., and Nadal, A. 2006. The estrogenic effect of bisphenol A disrupts pancreatic ??-cell function in vivo and induces insulin resistance. Environmental Health Perspectives 114(1): 106-112.
70. Nadal, A., Alonso-Magdalena, P., Soriano, S., Quesada, I., and Ropero, A.B. 2009. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implica- tions for blood glucose homeostasis and diabetes. Molecular and Cellular Endocrinology 304:63-68.
71. Bouchard, L., Thibault, S., Guay, S.P., Santure, M., Monpetit, A., St. Pierre, J., Perron, P., and Brisson, D. 2010. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care 33(11): 2436 ??? 2441.
72. Savastano, S., Tarantino, G., D???Esposito, V., Passaretti, F., Cabaro, S., Liotti, A., Liguoro, D., Perruolo, G., Ariemma, F., Finelli, C., Bequinot, F., Formisano, P., and Valentino, R. 2015. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. Journal of Translational Medicine 13(1): 1-7.
METABOLIC
73. Ellero-Simatos, S., Claus, S.P., Benelli, C., Forest, C., Letourneur, F., Cagnard, N., Beaune, P.H., and de Waziers, I. 2011. Combined Transcriptomic???1H NMR Metabonomic Study Reveals That Monoethylhexyl Phthalate Stimulates Adipogenesis and Glyceroneogenesis in Human Adipocytes. Journal of Proteome Research 10(12): 5493-5502.
74. Hofmann, P.J., Schomburg, L., and K??hrle, J. 2009. Interference of endocrine disrupters with thyroid hormone receptor-dependent transactivation. Toxicological Sciences 110(1): 125-137.
75. Marmugi, A., Ducheix, S., Lasserre, F., Polizzi, A., Paris, A., Priymenko, N., Bertrand-Michel, J., Pineau, T., Guillou, H., Martin, P.G., and Mselli-Lakhal, L. 2012. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 55(2): 395-407.
76. Schmutzler, C., Bacinski, A., Gotthardt, I., Huhne, K., Ambrugger, P., Klammer, H., Schlecht, C., Hoang-Vu, C., Gruters, A., Wuttke, W., Jarry, H., and K??hrle, J. 2007. The UV filter benzophenone 2 interferes with the thyroid hormone axis in rats and is a potent in vitro inhibitor of human recombinant thyroid peroxidase. Endocrinology 115(Suppl. 1): 77???83.
77. Hugo, E.R., Brandebourg, T.D., Woo, J.G., Loftus, J., Alexander, J.W., and Ben-Jonathan, N. 2008. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environmental Health Perspectives 116: 1642-1647.
NEUROLOGICAL
78. Fang, F., Chen, D., Yu, P., Qian, W., Zhou, J., Liu, J., Gao, R., Wang, J., and Xiao, H. 2015. Effects of Bisphenol A on glucose homeostasis and brain insulin signaling pathways in male mice. General and Comparative Endocrinology 212: 44-50.
79. El-Missiry, M.A., Othman, A.I., Al-Abdan, M.A., and El-Sayed, A.A. 2014. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. Journal of the Neurological Sciences 347(1): 251-256.
80. Kundakovic, M., and Champagne, F.A. 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain, Behavior, and Immunity 25(6): 1084-1093.
81. Hofmann, P.J., Schomburg, L., and K??hrle, J. 2009. Interference of endocrine disrupters with thyroid hormone receptor-dependent transactivation. Toxicological Sciences 110(1): 125-137.
82. Testa, C., Nuti, F., Hayek, J., De Felice, C., Chelli, M., Rovero, P., Latini, G., and Papini, A.M. 2012. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro 4(4): 223-229.
83. Clark-Taylor, T., and Clark-Taylor, B.E. 2004. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial ??-oxidation by long chain acyl-CoA dehydrogenase. Medical Hypotheses 62(6): 970-975.
84. Fang, L., Wuptra, K., Chen, D., Li, H., Huang, S.-K., Jin, C., and Yokoyama, K. K. 2014. Environmental-stress-induced Chromatin Regulation and its Heritability. Journal of Carcinogenesis & Mutagenesis, 5(1), 22058.
REPRODUCTIVE
85. Hannon, P.R., Peretz, J., and Flaws, J. 2014. Daily exposure to Di (2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biology of Reproduction 90(6): 136.
86. Hannon, P.R., and Flaws, J.A. 2015. The effects of phthalates on the ovary. Frontiers in Endocrinology 6:8.
87. Le??n-Olea, M., Martyniuk, C.J., Orlando, E.F., Ottinger, M.A., Rosenfeld, C.S., Wolstenholme, J.T., and Trudeau, V.L. 2014. Current concepts in neuroendocrine disruption. General and Comparative Endocrinology 203: 158-173.
88. Meeker, J.D., and Ferguson K.K. 2014. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011???2012. The Journal of Clinical Endocrinology & Metabolism 99(11): 4346-4352
89. Hannon, P. R., Peretz, J., and Flaws, J. A. 2014. Daily Exposure to Di(2-ethylhexyl) Phthalate Alters Estrous Cyclicity and Accelerates Primordial Follicle Recruitment Potentially Via Dysregulation of the Phosphatidylinositol 3-Kinase Signaling Pathway in Adult Mice. Biology of Reproduction 90(6), 136.
90. Braun, J.M., Just, A.C., Williams, P.L., Smith, K.W., Calafat, A.M., and Hauser, R. 2014. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. Journal of Exposure Science and Environmental Epidemiology 24(5): 459-466.
91. Soares, A., Guieysse, B., Jefferson, B., Cartmell, E., and Lester, J.N. 2008. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environment International 34(7): 1033-1049.
92. Lyche, J.L., Gutleb, A.C., Bergman, ??., Eriksen, G.S., Murk, A.J., Ropstad,Lyche, J.L., Gutleb, A.C., Bergman, ??., Eriksen, G.S., Murk, A.J., Ropstad, E., Saunders, M., and Skaare, J.U. 2009. Reproductive and developmental toxicity of phthalates ??? a review. Journal of Toxicololgy and Environmental Health B Critical Reviews 12(4): 225-249.
93. Wetherill, Y.B., Akingbemi, B.T., Kanno, J., McLachlan, J.A., Nadal, A., Sonnenschein, C., Watson, C.S., Zoeller, R.T., and Belcher, S.M. 2007. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology 24(2): 178-198.
94. Vega, A., Baptissart, M., Caira, F., Brugnon, F., Lobaccaro, J.-M. A., and Volle, D. H. 2012. Epigenetic: a molecular link between testicular cancer and environmental exposures. Frontiers in Endocrinology 3: 150.
DEVELOPMENTAL
95. Resendiz, M., Mason, S., Lo, C.-L., and Zhou, F. C. 2014. Epigenetic regulation of the neural transcriptome and alcohol interference during development. Frontiers in Genetics 5: 285.
96. Mason, S., and Zhou, F. C. 2015. Editorial: Genetics and epigenetics of fetal alcohol spectrum disorders. Frontiers in Genetics 6: 146.
97. Kim, J.H., Sartor, M. A., Rozek, L.S., Faulk, C., Anderson, O.S., Jones, T.R., Nahar, M.S., and Dolinoy, D.C. 2014. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics 15:30.
98. Walker, C. L. 2011. Epigenomic Reprogramming of the Developing Reproductive Tract and Disease Susceptibility in Adulthood. Birth Defects Research. Part A, Clinical and Molecular Teratology 91(8), 666???671.
99. Vega, A., Baptissart, M., Caira, F., Brugnon, F., Lobaccaro, J.-M. A., and Volle, D. H. 2012. Epigenetic: a molecular link between testicular cancer and environmental exposures. Frontiers in Endocrinology 3: 150.
100. Zhang, Z., Chen, S., Feng, Z., and Su, L.J. 2015. Pregnancy Exposures Determine Risk of Breast Cancer in Multiple Generations of Offspring. In: Environmental Epigenetics. Springer London. pp. 75-103.
101. Cao, J., Rebuli, M.E., Rogers, J., Todd, K.L., Leyrer, S.M., Ferguson, S.A., and Patisaul, H.B. 2013. Prenatal Bisphenol A Exposure Alters Sex-Specific Estrogen Receptor Expression in the Neonatal Rat Hypothalamus and Amygdala. Toxicological Sciences 133(1), 157???173.
102. Crinnion, W.J. 2010. Toxic effects of the easily avoidable phthalates and parabens. Alternative Medicine Review 15(3): 190-196.
ALLERGIES
103. Wang, I.-J., Karmaus, W. J., Chen, S.-L., Holloway, J. W., and Ewart, S. 2015. Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clinical Epigenetics 7(1): 27.
104. Dodson, R.E., Nishioka, M., Standley, L.J., Perovich, L.J., Brody, J.G., and Rudel, R.A. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental Health Perspectives 120(7): 935.
105. Hoppin, J.A., Jaramillo, R., London, S.J., Bertelsen, R.J., Salo, P.M., Sandler, D.P., Zeldin, D.C. 2013. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005???2006. Environmental Health Perspectives 121: 1129???1134.
106. Markey, C.M., Wadia, P.R., Rubin, B.S., Sonnenschein, C., and Soto, A.M. 2005. Long-term effects of fetal exposure to low doses of the xenoestrogen Bisphenol-A in the female mouse genital tract. Biology of Reproduction 72: 1344-1351.
107. Benachour, N., and Aris, A. 2009. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicology and Applied Pharmacology 241: 322-328.
108. LaPensee, E.W., Tuttle, T.R., Fox, S.R., and Ben-Jonathan, N. 2009. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-??-positive and ???negative breast cancer cells. Environmental Health Perspectives 117(2): 175-180.
109. vom Saal, F.S., and Welshons, W.V. 2006. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research 100: 50-76.
110. Baldi, E., and Muratori, M. 2013. Genetic damage in human spermatozoa. Advances in Experimental Medicine and Biology 791. New York. Springer-Verlag. 195p.
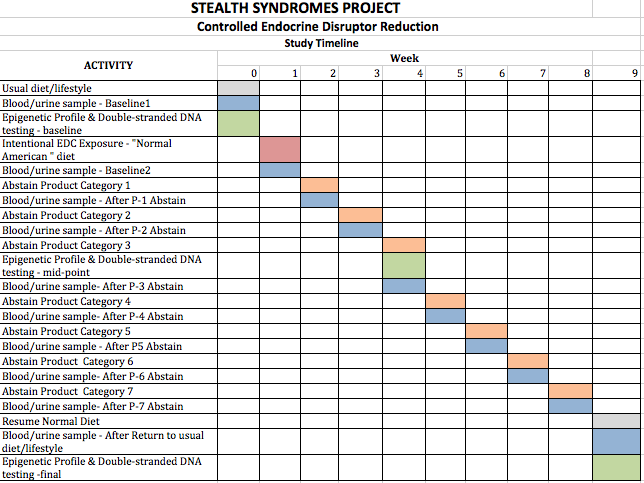
15.1 PROCEDURES/METHODS
- Weekly blood profile as direct clinical health-linked proxies for CEC body burden.
- Weekly blood profile and urine measurements of Bisphenol A and phthlates for correlation with blood biomarkers.
- Monthly double-stranded DNA break levels.
- Monthly epigenetic profiles of specific methylation locations known to be associated with cancer, obesity, aging, infertility, or Alzheimer???s disease.
We propose using specific elements of standard blood profiles that can provide direct health assessments of inflammation, glucose tolerance, lipid and cholesterol levels and similar well-established indicators.
Rationale For Selection of Specific Clinical Blood Tests
Preliminary universe of tests based on known cellular and biological mechanisms of BPA, phthalates and other CECs. To be narrowed down in consultation with a qualified hematologist.
- Estrogenic activity
- Anti-androgenic activity
- Oxidative stress / inflammation
- Glucose metabolism
- Insulin resistance
- Adipocyte functioning
- Epigenetic alterations
- Interference with Mitosis (centrioles)
- CDK5 effects (thyroid cancer)
- Prostate cancer (PSA levels)
- Accelerated cell proliferation and decreased apoptosis
- Affects on G-Protein Coupled Receptors
- WBC
- Cytokines:Il-1,6,8,10
- TNF alpha
- CRP
- BDNF, VEGF,IGF-BP#3,EGF,FGF,FGF-2?,NGF
- ESR
- F2 isoprostanes
- Cholesterol/HDL/triglycerides
- Hormones:cortisol,prolactin,GH,adiponectin,ghrelin,leptin,insulin, fasting glucose,NPY
- Vit E,C,D
- Fibrinogen
- Cell adhesion molecules:VCAM-1,ICAM
- Oxidative stress markers:glutthione peroxidase,superoxide dismutase,nitric oxide,
- Human methylation 450 bead chip
- telomere length; telomerase
Bisphenol A & Phthlates As Markers For Chemicals Of Concern
Any given product may contain multiple compounds, which makes the task of identifying which compounds (or synergistic combinations) are responsible for a given health effect impractical for this study.
Indeed, given the lack of data on the health effects of most chemicals involved, the task would be impossible for the budgets and technical abilities of the most advanced laboratories. Significantly, even less data is known about combined health effects of the everyday mixtures to which consumers are exposed.
To make this study possible and yield the best possible data, the products chosen for stepwise abstention have been categorized primarily with an eye towards those with established and previously measured levels of BPA and phthalates.
Given that the now-well-studied BPA and phthalate compounds are often used together — and always used in combination with other polymers, resins, and product enhancement chemicals — we theorize that they are suitable markers for the presence of other ???bad actors.???
Significantly, any health effects that may be observed from our study will clearly reflect possible synergistic effects from combinations of chemicals since it is impossible for us to know exactly which compounds are in a given product.
Study Product Category Rationale
In addition to selecting products with BPA and phthalate markers, we have also categorized products by their method of exposure:
- Consumption – migration and leaching from packaging8
- Consumption – migration and leaching from preparation stressors: heat, microwaving, ultraviolet/sunlight exposure, use of suspected utensils, preparation and eating surfaces
- Skin contact, inhalation
- Consumption – inherent content as purchased – resulting from harvest and processing
Product Category 1: Food (migration and leaching from packaging)
- Eliminating all products packaged in cans and plastic.
- Use of fresh products when possible.
- Products packed in glass may be substituted.
- Plastic-wrapped dry foods (bread, pasta, etc)
- Plastic-wrapped wet fresh foods (veggies, cheese, meat)
- Plastic storage bags
- Milk, Cheese, dairy products
- Cutting boards
Product Category 2: Food (migration and leaching from preparation stressors)
- Foods with metalized plastic ???crisping??? surfaces (Hot Pockets, frozen pizza)
- Paper or plastic plates, glasses and cups
- Take-out and deli plastic containers of all sorts.
- Restaurant and fast food
- Frozen and similar convenience foods
Product Category 3 (Non-alcoholic beverages, migration and leaching from packaging)
- Filtered tap water versus unfiltered.
- Homes/Offices where the water supply comes via PVC or Pex plastics.
- Beverages in pouches, boxes and ???paper bottles???
- Water in hydration bladders like Camelbak
- Drip coffee maker and Keurig (plastic) as well as the Sodastream
Product Category 4 (skin contact/inhalation)
- Laundry detergents (phthalates, fragrances, surfactants)
- Dish and dishwasher soaps (same as laundry)
- Toothpaste (plastic tube) ??? alternative?
- Toothbrush ??? what are the bristles made of?
- Floss?
- Fitbits, plastic watch bands
- Gore-Tex and other waterproof coatings
- Paper currency
- Receipts
Product Category 5 (Alcoholic Beverages – Non-alcoholic beverages, migration and leaching from packaging, Ethanol known solvent for chemicals)
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
- Wine in plastic pouches/bottles/boxes
- Distilled spirits in glass versus plastic bottles.
- Wine and beer ???on tap???
Product Category 6 (Alcoholic Beverages in glass bottles).
Alcohol consumption limited to two five-ounce pours of 14% wine or the equivalent.
Product Category 7 (Dairy products: Consumption – inherent content as purchased – resulting from harvest and processing)
The present study will focus on dairy products as a category for its own abstain/intervention. This is because a recent study found an unexpected increase in phthalates especially in children. That study theorized this increase was due to their greater consumption of milk than adults. Investigators in that study theorize that the extensive use of plastics in the milk-production process was responsible for the phthalates increase despite the fact that milk was delivered in glass bottles. In fact, that study calculated that children were exposed to 183 micrograms/kg/day and noted that level was more than 9X higher than, the EPA oral reference dose of 20 micrograms/kg/day.
15.2 INTERVIEWS, QUESTIONNAIRES, AND/OR SURVEYS WILL BE ADMINISTERED OR FOCUS GROUPS WILL BE CONDUCTED:
Test subjects will engage in real-time, daily logging of everything they eat, drink use, or apply to their bodies and will involve the removal of one item category per week.
Because BPA and phthalates are cleared within 24 hours8,17,21,22, a weekly schedule should provide adequate time for clearance of BPA and phthalates
However, given the certainty of unknown chemicals and their uncertain clearance rates from the body, this period is uncertain.
Items chosen for removal will be selected according to peer-reviewed, published data measuring CECs in consumer products.
We theorize that removal of items known to contain or leach chemicals of concern will result in improvement of test subjects??? blood profiles as well as epigenetic profiles and double-stranded DNA break analysis
If that is confirmed, then it may be reasonable to conclude that the removal of those items was responsible.
It is possible that the change of chemical levels measured may fall beneath the noise level or the margins of error for an individual test. In those cases, we anticipate that the longer term levels will show a decrease.
16.4 BENEFITS TO SOCIETY
- First connection established between dietary intervention and health indicators.
- Establishment of a framework to move risk assessment of low-level Chemicals of Emerging Concern (CECs) beyond traditional toxicological evaluations toward molecular and epigenetic evaluations.
- Development of techniques to reduce exposure to CECs.
- Emphasis on techniques (#2, above) that can easily and economically be implemented by the average person without significant disruption to daily lives.
- Overall improvement in public health and a potential path to reducing the rising incidence of obesity, Type 2 Diabetes, Alzheimer’s disease and other behavioral disorders, fertility and developmental disorders.